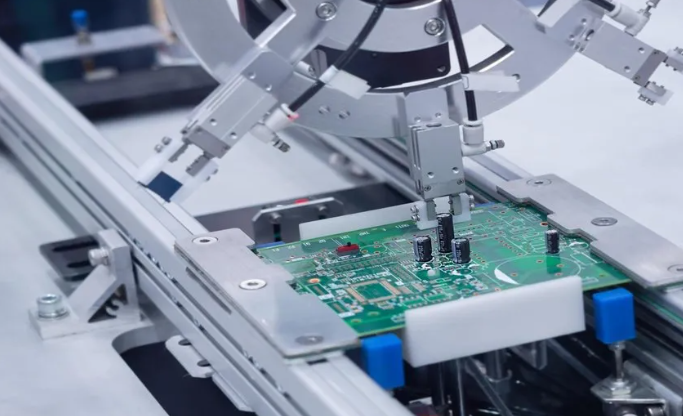
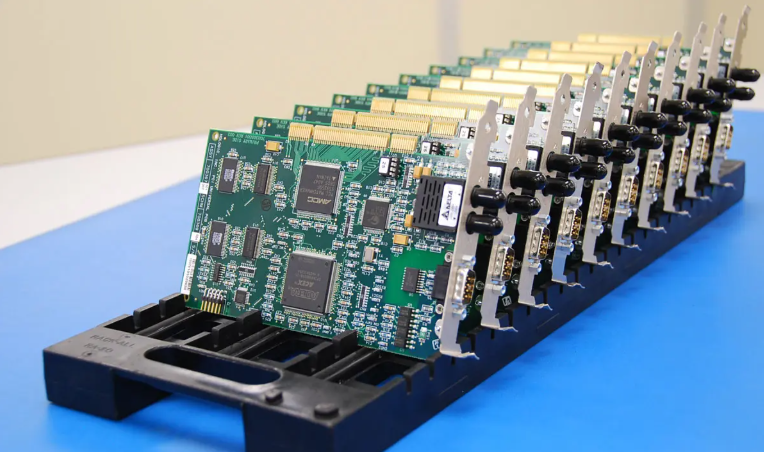
Medical PCBA‚Äč (Printed Circuit Board Assembly) forms the electronic heartbeat of modern medical equipment, from diagnostic monitors to life-sustaining implants. This article explores the specialized world of medical device electronics manufacturing, detailing the critical standards, design considerations, and supply chain strategies that ensure safety, reliability, and accelerated innovation.
Developing a new medical PCBA device is fraught with unique challenges. Do you struggle with these common hurdles?
- Navigating complex regulatory compliance (e.g., ISO 13485, FDA) for electronics.
- Achieving high reliability and longevity in critical, life-dependent applications.
- Sourcing biocompatible and high-performance materials for specialized designs.
- Managing extended lead times for multilayer medical PCBA production‚Äč and components.
- Validating design functionality and manufacturability early without slowing down.
An expert medical PCBA manufacturer‚Äč addresses these points directly.
- Providing ISO13485 medical PCBA‚Äč certified manufacturing and comprehensive documentation support.
- Implementing rigorous medical PCBA quality control‚Äč and testing protocols for high-reliability medical PCBA.
- Offering expertise in material selection for biocompatible medical PCBA‚Äč and high-performance medical PCBA‚Äč designs.
- Delivering quick turn PCBA prototype medical device‚Äč services to compress development cycles.
- Offering best turnkey PCB and PCBA small-batch services for medical devices‚Äč for seamless early-stage builds.
At EBest Circuit (Best Technology), we are a specialized medical device PCBA supplier‚Äč focused on medical PCBA design, custom medical PCBA prototypes, precision medical PCBA mass production, and medical PCBA testing. We have implemented an MES system at our factory. And we combine ISO 13485:2016 certified processes with rapid prototyping expertise to offer fast track PCBA medical‚Äč device development, helping you transition from medical PCBA design to ISO 13485 medical PCBA customized product with fast speed. A warm welcome to contact us at sales@bestpcbs.com‚Äč to discuss your OEM medical control PCBA assembly project.
What Is Medical PCBA?
Medical PCBA refers to the specialized process of assembling printed circuit boards for use in medical devices and equipment. This domain demands an extreme focus on precision, traceability, and reliability, as these electronic assemblies often perform critical diagnostic, monitoring, or therapeutic functions.
Unlike consumer electronics, medical PCBAs are characterized by:
- Stringent Regulatory Compliance:‚Äč Adherence to standards like ISO 13485 (Quality Management) and IEC 60601-1 (Safety) is non-negotiable.
- Enhanced Reliability Requirements:‚Äč Devices must perform flawlessly over long service lives, often in demanding environments.
- Material Rigor:‚Äč Use of high-Tg laminates, biocompatible coatings, and conformal coatings suitable for sterilization or bodily contact.
- Comprehensive Traceability:‚Äč Full documentation of components, materials, processes, and test results for each batch or unit.
- Robust Testing Regimens:‚Äč Subjecting assemblies to rigorous electrical, environmental, and functional tests beyond standard industrial practice.
In essence, medical PCBA‚Äč is the foundation upon which safe, effective, and innovative healthcare technology is built, requiring a manufacturing partnership that prioritizes quality and compliance above all else.

How Does PCBA Medical Device Manufacturing Differ From Standard Industrial PCBA?
The manufacturing of PCBA for medical equipment‚Äč operates under a fundamentally different paradigm than standard industrial assembly, primarily governed by risk management and patient safety. The key distinctions are systemic and profound.
| Aspect | Standard Industrial PCBA | Medical Device PCBA Manufacturing |
|---|---|---|
| Governance Standard | ISO 9001 Quality System | ISO 13485 Risk-Based QMS |
| Primary Driver | Cost, Performance, Speed | Safety, Compliance, Reliability |
| Traceability | Lot-Level Tracking | Full Batch or Unit Traceability |
| Documentation | BOM, Gerber, Assembly Files | DHF, DMR, Controlled Records |
| Cleanliness Control | IPC-Based Handling | Strict Contamination Control |
| Failure Impact | Downtime, Financial Loss | Patient Risk, Regulatory Exposure |
| Testing & Validation | Sample Testing | 100% Testing, IQ/OQ/PQ |
Therefore, choosing a partner experienced in medical electronics PCBA‚Äč is critical. They understand that every process‚ÄĒfrom medical PCBA fabrication‚Äč to final inspection‚ÄĒis part of a controlled, documented system aimed at ensuring the highest possible outcome for patient health.
What Materials And Standards Are Required For High-Reliability PCB Medical Designs?
Creating a high-reliability medical PCBA‚Äč starts with the foundational choices of materials and adherence to a strict hierarchy of standards. These choices directly impact the device’s performance, longevity, and regulatory acceptability.
Core Material Considerations:
- PCB Laminates:‚Äč High-Tg (Glass Transition Temperature) materials like FR-4 Tg170 or polyimide are standard for better thermal and mechanical stability during assembly and in-field use.
- Surface Finishes:‚Äč For PCB customized immersion gold bare PCBA board for medical equipment, Immersion Gold (ENIG) offers excellent planarity for fine-pitch components and reliable shelf life. Other options include Immersion Silver or hard gold for edge connectors.
- Conformal Coatings:‚Äč Biocompatible, moisture-resistant coatings (e.g., USP Class VI approved silicone or parylene) are often required for wearable medical PCBA‚Äč or devices exposed to fluids.
- Components:‚Äč Use of automotive-grade or higher-specification components to ensure tolerance to stress and long-term availability.
Essential Standards Framework:
- ISO 13485:2016:‚Äč The cornerstone quality management standard for medical device manufacturing.
- IEC 60601-1:‚Äč The international standard for basic safety and essential performance of medical electrical equipment.
- IPC-A-610 (Class 3):‚Äč The highest acceptability standard for electronic assemblies, mandatory for critical applications.
- ISO 14971:‚Äč Application of risk management to medical devices.
- FDA 21 CFR Part 820:‚Äč Quality System Regulation for devices marketed in the United States.
- EU MDR (2017/745):‚Äč Regulatory framework for devices in the European Union.
A proficient custom ISO13485 medical PCBA‚Äč partner guides this selection process, ensuring your medical PCB assembly‚Äč meets all material and regulatory prerequisites for a successful submission.
Why Is Multilayer Medical PCBA Production Important For Advanced Diagnostic Systems?
Multilayer medical PCBA production‚Äč is not just a technical choice but a necessity for the advanced functionality, speed, and miniaturization required in next-generation diagnostic systems like MRI machines, CT scanners, and digital PCR instruments.
The importance stems from several critical advantages:
- Increased Circuit Density:‚Äč Allows for more complex functionality in a smaller footprint, crucial for portable or handheld diagnostic tools.
- Improved Signal Integrity:‚Äč Dedicated power and ground planes reduce noise and cross-talk, which is vital for the high-speed, low-noise analog signals found in sensors and imaging detectors.
- Enhanced Thermal Management:‚Äč Internal layers can help dissipate heat from high-power components more effectively, improving reliability.
- Better EMI/RFI Shielding:‚Äč Carefully designed layer stack-ups can contain electromagnetic interference, preventing it from affecting sensitive measurements or violating emission regulations.
- Design Flexibility:‚Äč Enables the integration of mixed-signal (analog/digital/RF) circuits on a single board with proper isolation.
For a medical device PCBA manufacturer, producing these complex multilayer boards requires precision lamination, advanced laser drilling for microvias, and stringent impedance control. This capability is fundamental to delivering the precision medical PCBA at the core of devices that clinicians and patients depend on for accurate diagnoses.
How Can Wearable Medical PCBA Enable Next-Generation Patient Monitoring Devices?
Wearable medical PCBA‚Äč is the driving force behind the shift from episodic clinic-based care to continuous, personalized health monitoring. These PCBA designs present unique engineering challenges that, when solved, unlock transformative patient benefits.
Key enabling factors of wearable PCBA technology include:
- Ultra-Miniaturization:‚Äč Using HDI (High-Density Interconnect) techniques and chip-scale packaging to create tiny, unobtrusive devices.
- Low-Power Design:‚Äč Incorporating ultra-low-power MCUs, efficient power management ICs (PMICs), and energy harvesting possibilities to enable multi-day battery life.
- Flexible & Rigid-Flex PCBs:‚Äč Allowing the assembly to conform to the body’s contours, improving comfort and wearability for items like patches or smart clothing.
- Robust Connectivity:‚Äč Reliably integrating Bluetooth Low Energy (BLE), Wi-Fi, or cellular modems for seamless data transmission to cloud platforms.
- Sensor Fusion:‚Äč Precision medical PCBA‚Äč that accurately integrates multiple sensors (ECG, PPG, accelerometer, temperature) on a single, stable platform.
- Environmental Robustness:‚Äč Designs must be sweat-proof, dust-resistant, and capable of enduring daily mechanical stress, requiring careful medical PCBA design‚Äč and coating strategies.
By mastering these aspects, a medical electronics PCBA‚Äč specialist can help develop wearable medical PCBA‚Äč solutions that provide continuous vital sign monitoring, early anomaly detection, and improved patient outcomes outside traditional clinical settings.
Why Do Engineers Choose Prototype PCBA Medical Device Services For Early Validation?
Engineers opt for dedicated prototype PCBA medical device‚Äč services to de-risk the development process long before committing to full-scale production. This phase is about empirical validation and iterative refinement.
The primary reasons for this crucial step are:
- Functional Verification:‚Äč Testing the real-world performance of the circuit design, firmware, and sensor interfaces.
- Form Factor Testing:‚Äč Ensuring the physical PCB assembly fits within the intended industrial design and enclosure.
- Thermal & EMI Assessment:‚Äč Identifying hot spots or electromagnetic interference issues in a representative assembly.
- Manufacturability Analysis (DFM):‚Äč Uncovering potential production flaws‚ÄĒsuch as component placement issues or soldering defects‚ÄĒthat are not apparent in CAD models.
- Regulatory Strategy Testing:‚Äč Early identification of test points and data collection needs for future regulatory submissions.
- Stakeholder Demonstration:‚Äč Creating tangible units for internal reviews, investor pitches, or early clinician feedback.
A partner offering best turnkey PCB and PCBA small-batch services for medical devices‚Äč is ideal for this stage. They provide a seamless transition from design files to functional prototypes, incorporating necessary medical PCBA quality control‚Äč checks even at low volumes to ensure the prototype is a meaningful test article.
How Can Quick Turn PCBA Prototype Medical Device Reduce Time To Market?
Quick turn PCBA prototype medical device‚Äč services are a strategic accelerator, directly compressing the critical path of medical device development and directly addressing the need for fast track PCBA medical‚Äč programs.
The time-saving impact is realized across multiple phases:
- Shortened Design Cycles:‚Äč Rapid feedback from physical prototypes allows for faster design iterations, moving from “what if” to “what is” in days, not weeks.
- Parallel Development:‚Äč Hardware prototypes can be built and tested while software is being developed, and while enclosure tooling is being designed.
- Faster Regulatory Testing:‚Äč Having stable, production-representative prototypes earlier allows regulatory testing (safety, EMC, biocompatibility) to begin sooner.
- Supply Chain Validation:‚Äč Prototype builds help verify component availability and performance, preventing last-minute sourcing crises during ramp-up.
- Early User Feedback:‚Äč Functional prototypes enable crucial human factors and usability testing, leading to design improvements before costly tooling is finalized.
For a China electronics PCBA supplier for medical equipment with ISO13485, offering reliable quick-turn services while maintaining medical-grade processes is a key competitive advantage. It allows global innovators to leverage efficient manufacturing without compromising on the rigorous standards required for medical device PCBA manufacturing.
How Does Medical PCBA Quality Control Ensure Long-Term Reliability?
Medical PCBA quality control‚Äč is a multi-layered, continuous process designed to ensure that every single assembly will perform its intended function reliably over its entire specified service life. It goes far beyond simple visual inspection.
A comprehensive QC regimen includes:
- Incoming Material Inspection:‚Äč Certifying all components and PCB bare boards to required specifications.
- Automated Optical Inspection (AOI):‚Äč 100% inspection for soldering defects, component presence, and correct placement.
- X-Ray Inspection (AXI):‚Äč Essential for checking hidden solder joints under BGAs or in multilayer medical PCBA production.
- In-Circuit Test (ICT):‚Äč Verifies component values, presence, and basic connectivity.
- Functional Testing (FCT):‚Äč Simulates the device’s operating environment to validate full assembly performance.
- Environmental Stress Screening (ESS):‚Äč Subjecting units to thermal cycling or burn-in to precipitate early-life failures.
- Data Recording & Traceability:‚Äč Documenting every test result and linking it to the specific unit and its component batches.
This rigorous approach, mandated by standards like ISO 13485, is what defines a true high-reliability medical PCBA‚Äč supplier. It transforms quality from a final checkpoint into a property built into the product at every stage.
What Should You Evaluate When Choosing A Medical Device PCBA Manufacturer?
Selecting the right medical device PCBA manufacturer‚Äč is a decision that impacts your product’s safety, success, and scalability. The evaluation must extend beyond basic capabilities to encompass culture and systems.
Critical evaluation criteria include:
- Certifications & Regulatory Expertise:‚Äč Valid ISO 13485:2016‚Äč certification is the baseline. Experience with FDA audits and EU MDR is a major plus.
- Quality Systems & Traceability:‚Äč Assess their documentation practices, lot/unit traceability systems, and approach to corrective and preventive actions (CAPA).
- Technical & Material Competence:‚Äč Evaluate their experience with your specific technology (HDI, flex, RF) and materials (biocompatible coatings, high-reliability laminates).
- Prototyping & NPI Process:‚Äč Scrutinize their quick turn PCBA prototype medical device‚Äč process and New Product Introduction (NPI) workflow for efficiency and feedback quality.
- Supply Chain Resilience:‚Äč Understand their component sourcing strategies, relationships with distributors, and management of long-lead-time items.
- Communication & Transparency:‚Äč The partner must act as an extension of your team, providing clear, proactive communication, especially when issues arise.
- Scalability:‚Äč Ensure they can support you from prototype PCBA medical‚Äč builds through to OEM PCBA for medical equipment‚Äč volume production.
What Makes A China Electronics PCBA Supplier For Medical Equipment With ISO13485 Competitive Globally?
A China electronics PCBA supplier for medical equipment with ISO13485‚Äč competes globally by offering an unmatched combination of stringent quality, advanced technical capability, and scalable efficiency.
The formula for global competitiveness is:
- Deep Process Rigor, Not Just Certification:‚Äč Truly integrating the risk-management principles of ISO 13485 into every workflow, from medical PCBA design‚Äč to shipping, creating a culture of quality equal to Western counterparts.
- Advanced Manufacturing Infrastructure:‚Äč Investing in state-of-the-art SMT lines, precision assembly equipment, and comprehensive testing labs (AOI, X-Ray, FCT) capable of producing high-performance China medical PCBA.
- Integrated Supply Chain & Cost Efficiency:‚Äč Proximity to the world’s largest component and raw material markets enables stable sourcing and significant cost advantages without sacrificing quality.
- Technical Engineering Support:‚Äč Providing valuable medical PCBA services‚Äč like DFM/DFA analysis, material selection guidance, and test fixture design, adding engineering value beyond simple assembly.
- Flexibility and Speed:‚Äč Excelling at fast track PCBA medical device‚Äč prototyping and supporting rapid design changes, which accelerates the overall innovation cycle for global clients.
By mastering this blend, leading China medical PCBA suppliers like EBest Circuit (Best Technology) transition from being seen as low-cost vendors to being strategic partners capable of delivering custom medical PCBA‚Äč solutions that are reliable, compliant, and cost-effective for the global market.
In a nutshell, medical PCBA‚Äč is the critical, behind-the-scenes technology that powers the safety, intelligence, and innovation of modern healthcare devices. Success in this field demands a manufacturing partnership that equally prioritizes unwavering quality, regulatory intelligence, and agile development support.
Navigating the journey from a prototype to a certified, reliable product requires a partner with a proven system. EBest Circuit (Best Technology) provides exactly that. As your dedicated medical device PCBA supplier, we combine our ISO 13485:2016 certified processes with expertise in quick turn PCBA prototype medical device‚Äč development and high-reliability medical PCBA‚Äč production. Let us help you accelerate your time to market while ensuring the utmost quality and compliance. Contact our team today at sales@bestpcbs.com‚Äč to start a conversation about your next medical electronics project.
FAQs about Medical PCBA
What is a medical PCB?
A medical PCB is the bare, unpopulated printed circuit board designed specifically for use in a medical device. It is characterized by the use of high-reliability materials (like high-Tg laminates), stringent tolerance controls, and often specialized features like impedance control or biocompatible surface finishes. It serves as the foundational substrate for the medical PCB assembly‚Äč process.
How Do Engineers Verify Quality When Outsourcing Medical PCBA Manufacturing?
Engineers verify quality through a multi-faceted approach:
First, they audit the supplier’s ISO13485 medical PCBA certification and quality management system.
Second, they review the supplier’s detailed Quality Control plan, insisting on 100% electrical testing and comprehensive inspection reports (AOI, X-Ray).
Third, they conduct regular on-site audits (or virtual audits) and perform rigorous acceptance testing on incoming batches.
Finally, they establish clear quality agreements that define roles, responsibilities, and metrics for failure analysis and corrective actions.
Why Is Traceability So Important In Medical PCB Assembly?
Traceability is paramount in medical PCB assembly‚Äč for three critical reasons:
1) Patient Safety:‚Äč In the event of a component failure or field issue, full unit-level traceability allows for the precise and rapid identification of all affected devices, enabling targeted recalls to prevent patient harm.
2) Regulatory Requirement:‚Äč Standards like ISO 13485 and FDA 21 CFR Part 820 explicitly require traceability of components, materials, and production processes.
3) Process Control & Improvement:‚Äč Traceability data helps isolate the root cause of production defects, enabling effective corrective actions and continuous improvement of the manufacturing process.