Yellow discoloration on Al₂O₃ DBC substrates layers is occasionally observed after DBC bonding. For many engineers, this visual change raises a practical question: does ceramic yellowing indicate a performance risk? Why does it become yellow? This article explains the material mechanisms behind discoloration, and why it does not compromise DBC substrate reliability.
What Is an Al₂O₃ DBC Substrate?
An Al₂O₃ DBC (Direct Bonded Copper) substrate is a ceramic metal composite formed by directly bonding copper foil to an alumina ceramic plate under high temperature and controlled oxygen atmosphere.
This structure is widely used in power modules, inverters, IGBT packages, and automotive electronics, where thermal conductivity and electrical insulation are critical. The bonding process relies on interfacial chemical reactions, not adhesives.

How Does the DBC Bonding Process Work?
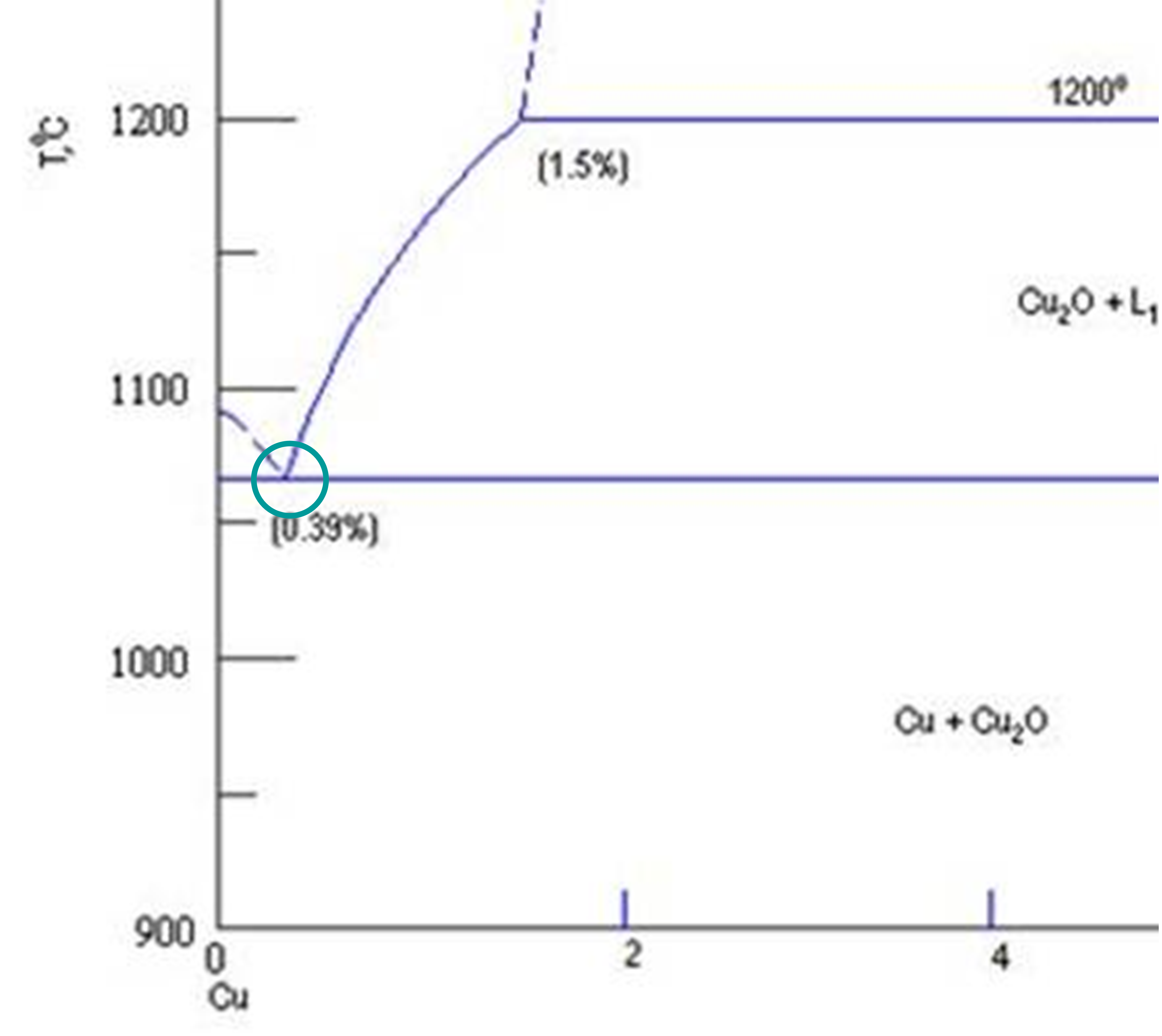
During DBC sintering, copper and alumina interact through a thermally activated oxidation-reduction mechanism.
At elevated temperatures and controlled oxygen levels:
- Copper surface oxidizes to form a thin Cu₂O layer
- When the temperature exceeds the eutectic point, a Cu–Cu₂O eutectic liquid phase forms
- This liquid phase wets both copper and Al₂O₃ surfaces effectively
- Copper oxide reacts with alumina to form CuAlO₂
The reaction can be summarized as:
Cu₂O + Al₂O₃ → CuAlO₂

CuAlO₂ acts as a ceramic-like bonding phase, enabling strong metallurgical adhesion between copper and ceramic.
What Is the Bonding Layer Made Of?
After bonding, a thin interfacial layer composed mainly of CuAlO₂ exists between copper and Al₂O₃.
Key characteristics of this layer:
- Ceramic oxide with dark red to yellowish color
- Excellent resistance to acids and alkalis
- Difficult to remove completely using standard copper etching chemicals
When surface copper is etched away, the bonded ceramic area often appears more yellow than unbonded areas. This visual difference directly relates to the presence of the bonding layer.
Why Does Yellow Discoloration Appear on the Ceramic Surface?
Yellowing originates from two simultaneous material phenomena, both occurring during high-temperature DBC sintering.
1. Influence of the CuAlO₂ Bonding Layer
CuAlO₂ itself has a reddish-yellow ceramic appearance. Because this compound forms through interfacial reactions, its thickness is not perfectly uniform across the substrate.
Even nanometer-level thickness variation can lead to visible color differences after copper removal.

2. Migration of Ceramic Sintering Additives
Most industrial Al₂O₃ ceramics used for DBC are 96% alumina, not 100% pure.
They contain small amounts of sintering additives such as:
- SiO₂
- CaO
- MgO
These additives improve ceramic densification during firing.
During DBC bonding:
- High temperatures cause partial diffusion of these additives toward the surface
- EDX analysis shows increased Si, Ca, and Mg content in yellowed areas
- Higher surface concentration of these oxides correlates with yellow coloration
This behavior is consistent with ceramic sintering literature, which confirms that increased SiO₂ content can cause alumina yellowing.
How Does the DBC Bonding Process Create a Strong Interface?
DBC bonding relies on controlled oxidation and eutectic reactions at high temperature.
During sintering:
- Copper forms a thin Cu₂O layer under controlled oxygen content
- Above the eutectic temperature, a Cu–Cu₂O liquid phase appears
- This liquid wets both copper and Al₂O₃ surfaces
- Interfacial reactions form CuAlO₂, enabling direct bonding
This mechanism creates a chemically bonded interface, which is critical for long-term thermal cycling stability.
Why Is the Yellow Color Often Non-Uniform?
The discoloration is rarely perfectly even. This non-uniformity comes from reaction uncertainty, not process instability.
Key reasons include:
- CuAlO₂ formation varies slightly across the bonding interface
- Sintering additive diffusion is not perfectly uniform at the micro-scale
- Both effects occur at nanometer-level thickness differences
Even minor variations become visible on ceramic surfaces due to light reflection and oxide color sensitivity.
Does Yellow Discoloration Affect DBC Substrate Performance?
This is the most important question for engineers and customers. Extensive testing was performed on:
- DBC substrates with severe yellow discoloration
- DBC substrates with minimal or no discoloration
The results show:
- No meaningful difference in electrical insulation
- No degradation in thermal performance
- No impact on mechanical bonding strength
- No reliability concerns in functional testing
In short, yellow discoloration is a cosmetic phenomenon, not a functional defect.

What Is an Al₂O₃ DBC Substrate Used For?
Al₂O₃ DBC substrates are widely applied in:
- IGBT and power module packaging
- Automotive inverters and motor drives
- Industrial power supplies
- Renewable energy systems
Their value lies in a balanced combination of electrical insulation, thermal conduction, and mechanical stability. The copper–ceramic interface is formed through a metallurgical bonding process rather than adhesives or plating.
How EBest Circuit (Best Technology) Controls DBC Substrate Quality?
At EBest Circuit (Best Technology), DBC substrates are produced with strict control over:
- Oxygen concentration during bonding
- Temperature uniformity across furnaces
- Ceramic material composition and sourcing
- Post-bonding inspection and testing
Our engineering team evaluates DBC substrates based on measurable electrical and thermal performance, not cosmetic appearance alone.
This approach ensures stable results for:
- Automotive-grade power electronics
- Industrial and renewable energy systems
- High-reliability inverter applications
Conclusion
Yellow discoloration on Al₂O₃ DBC substrates originates from:
- CuAlO₂ formation at the bonding interface
- Surface diffusion of ceramic sintering additives
Both effects occur during normal DBC sintering and may vary slightly across the substrate.
Extensive testing confirms that this discoloration does not affect DBC performance or reliability.
For power electronics applications, engineering performance matters far more than visual color uniformity.


