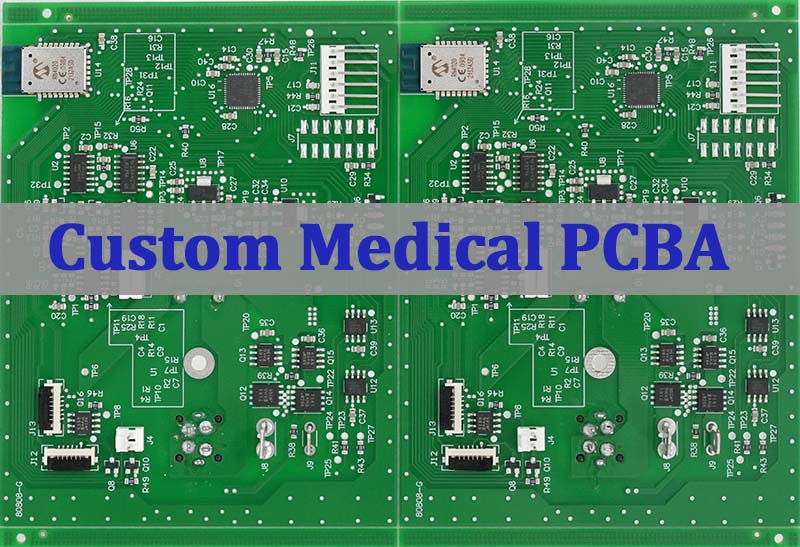
Custom Medical PCBA plays a critical role in modern healthcare electronics, where PCB reliability, PCBA traceability, and regulatory compliance are non-negotiable. From patient monitoring systems to diagnostic imaging equipment, medical devices demand precise SMT PCB assembly processes supported by strict quality systems such as ISO13485 and IPC Class 3 standards. This guide explains how engineers and procurement teams can evaluate suppliers, understand design requirements, and ensure long-term stability when developing custom PCBA for regulated medical applications.
Unlike consumer electronics, medical PCBA projects involve higher documentation standards, tighter process control, and extended product life cycles. EBest Circuit (Best Technology) delivers high-reliability custom medical PCBA solutions in line with ISO 13485 standards, supporting medical and high-precision electronic applications with strict quality control and full traceability. By working closely with customers, the engineering team helps clients have a better picture of manufacturability risks while giving them easy access to professional DFM feedback and global supply chain resources. This collaborative approach provides a strong head start during product development and helps heighten overall reliability, efficiency, and compliance from prototype stages through mass production. For more information or inquiries, please contact us via the form on our Contact page.
What Defines A Reliable Custom Medical PCBA Manufacturer For Regulated Healthcare Devices?
A dependable manufacturer must demonstrate more than basic SMT capability. Medical electronics require strict process discipline and full lifecycle management.
Key characteristics include:
- ISO13485-certified quality management systems
- Full traceability for components and manufacturing batches
- Controlled ESD environments and clean assembly conditions
- Advanced inspection technologies such as AOI, AXI, and functional testing
Reliability is closely linked to process consistency. A reliable partner maintains standardized work instructions, validated reflow profiles, and strict material control procedures. These elements reduce the risk of latent defects that could compromise patient safety.
In addition, experienced manufacturers support early-stage DFM reviews. By identifying potential issues such as pad geometry or thermal imbalance, they help engineers prevent costly redesigns later in the project lifecycle.
What Design Rules Should Engineers Follow When Building A Custom Medical PCBA For Diagnostic Devices?
Designing PCBA for diagnostic equipment requires balancing electrical performance with regulatory constraints. Engineers typically follow conservative design principles to ensure long-term stability.
Important design considerations include:
- Using medical-grade components with long lifecycle availability
- Maintaining adequate creepage and clearance distances
- Implementing redundant grounding paths for noise-sensitive circuits
- Selecting high-reliability surface finishes such as ENIG or ENEPIG
Thermal management is another major factor. Diagnostic systems often run continuously, which increases the risk of thermal fatigue. Designers must optimize copper distribution, via structures, and component spacing to maintain stable operating temperatures.
Documentation also forms part of the design process. Detailed fabrication drawings, BOM traceability, and revision control are essential to comply with regulatory audits.
How Does PCB Customized Immersion Gold Bare PCBA Board For Medical Equipment Enhance Long-Term Reliability?
Immersion gold surface finishes, commonly known as ENIG, are widely used in medical electronics due to their excellent corrosion resistance and stable solderability. A pcb customized immersion gold bare pcba board for medical equipment helps maintain consistent electrical performance over extended product lifetimes.
Advantages include:
- Flat surface suitable for fine-pitch components and BGAs
- Strong resistance to oxidation during storage
- Improved contact reliability for connectors and test points
For devices exposed to repeated sterilization or harsh environments, ENIG reduces the risk of surface degradation. The nickel layer acts as a diffusion barrier, while the gold layer provides stable conductivity.
However, process control is critical. Improper plating thickness or poor bath management can lead to black pad issues. Therefore, manufacturers must maintain strict chemical monitoring and inspection routines.
What Testing And Validation Steps Are Required For Custom ISO13485 Medical PCBA Before Mass Production?
Medical PCBA projects typically undergo extensive validation before entering full production. These procedures ensure compliance with regulatory requirements and confirm product reliability.
Common testing stages include:
- Design Verification Testing (DVT) to confirm electrical performance
- Process Qualification to validate SMT parameters
- Functional testing under simulated operating conditions
- Environmental stress screening such as thermal cycling
Manufacturers following ISO13485 standards also maintain structured documentation, including IQ/OQ/PQ reports and traceability records. These documents support regulatory submissions and provide evidence of process consistency.
Early testing reduces the risk of field failures and helps identify potential design weaknesses before mass production begins.
How To Evaluate A Supplier Offering Custom ISO13485 Medical PCBA For USA Medical Projects?
When sourcing a custom ISO 13485 medical pcba partner, engineers should assess both technical capability and regulatory readiness.
Evaluation criteria may include:
- Experience with FDA-regulated products
- Availability of in-house engineering support
- Capability to handle multilayer and HDI PCB structures
- Traceability systems such as MES or barcode tracking
Communication is equally important. A responsive engineering team can quickly address design changes, component shortages, or compliance questions, ensuring smoother project execution.
Additionally, suppliers should provide transparent documentation processes. Clear reporting builds trust and simplifies audits during product certification stages.
When Should You Consider China Custom-Made ISO13485 Multilayer Medical Electronics Interface PCBA?
Global supply chains often combine USA design expertise with advanced manufacturing resources from overseas. Choosing China custom-made ISO13485 multilayer medical electronics interface pcba may be beneficial in several scenarios.
These include:
- Projects requiring complex multilayer PCB fabrication
- High-volume production with strict cost targets
- Advanced assembly processes such as microvia HDI or fine-pitch placement
Collaborating with experienced international manufacturers allows OEMs to access mature supply chains and specialized equipment. However, successful collaboration depends on strong quality management and transparent communication channels.
Many USA-based companies adopt a hybrid model, where early prototyping and design validation occur locally while mass production leverages global manufacturing capacity.

In closing, custom medical PCBA manufacturing requires a careful balance between engineering precision and regulatory compliance. From design rules to testing validation and supply chain selection, every stage plays a role in ensuring safe and reliable medical devices. By working with experienced manufacturers that understand ISO 13485 processes and advanced PCB assembly techniques, healthcare companies can accelerate product development while maintaining strict quality standards.
Whether you are developing diagnostic PCBA equipment, wearable medical PCBA electronics, or advanced imaging PCBA systems, choosing the right manufacturing custom ISO 13485 medical PCBA partner helps reduce risk, improve product longevity, and ensure compliance with global healthcare regulations. Feel free to reach out to us at sales@bestpcbs.com for any technical questions or project discussions.
FAQs About Custom Medical PCBA
Do Custom Medical PCBA Suppliers Need To Hold ISO13485 Certification?
Yes. ISO13485 certification demonstrates that a manufacturer follows structured quality management processes tailored for medical device production. While additional standards may apply depending on the product category, ISO13485 is widely considered the baseline requirement for regulated healthcare electronics.
What Documentation Should Engineers Request From A Custom Medical PCBA Supplier Before Production?
Engineers should request process flow charts, inspection reports, material certifications, and traceability records. Documentation such as DFM analysis, validation reports, and quality control plans helps ensure that the supplier meets regulatory expectations.
How Do Engineers Verify Whether A Custom Medical PCBA Supplier Is Truly Compliant?
Compliance can be verified through on-site audits, certification checks, and reviewing process documentation. Engineers often evaluate whether the supplier maintains consistent testing procedures, documented work instructions, and controlled production environments aligned with ISO13485 standards.
Tags: China Custom-Made ISO13485 Multilayer Medical Electronics Interface PCBA, Custom ISO13485 Medical PCBA, Custom Medical PCBA, Medical Custom PCBA, PCB Customized Immersion Gold Bare PCBA Board For Medical Equipment, pcba custom medical


