What is IQ in Medical Terms?
Installation Qualification (IQ) is the documented verification that all equipment, systems, and infrastructure used in medical PCBA manufacturing are installed correctly and conform to approved specifications.
In regulated medical electronics, IQ is not a procedural formality; it is the foundation of process validation.
Before evaluating solder quality, yields, or test results, manufacturers must first prove that the manufacturing environment itself is controlled, traceable, and suitable for medical production. IQ provides this proof in a structured and auditable manner.
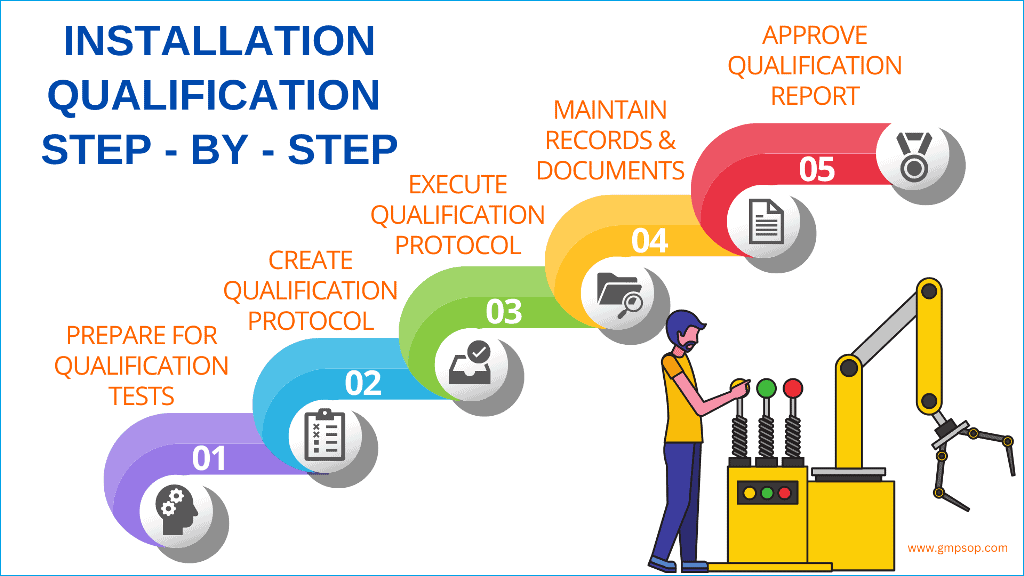
Why Is IQ the First Step in Medical PCBA Process Validation?
Medical regulations emphasize process control over end-product inspection. A compliant output cannot compensate for an uncontrolled environment.
IQ is performed first because it:
- Establishes baseline manufacturing conditions
- Confirms equipment configuration and utilities
- Locks approved software and hardware versions
- Creates traceable records for future audits
Without IQ, subsequent validation activities such as OQ and PQ lack regulatory credibility.
Which Equipment and Systems Are Included in Medical PCBA IQ?
At Best Technology, IQ for medical PCBA projects typically covers:
- SMT pick-and-place machines, including placement accuracy and software revision
- Solder paste printing systems and stencil alignment capability
- Reflow and curing ovens, including zone configuration and atmosphere control
- AOI, SPI, and X-ray inspection systems
- Electrical and functional test platforms
- ESD protection infrastructure
- MES-based traceability systems
- Calibration tools and measurement equipment
Each item is verified against predefined installation requirements, including environmental conditions, utilities, configuration, and calibration status.
Why Is IQ Required for Medical PCBA Compliance?
Medical regulations such as ISO 13485 emphasize process control over final inspection. IQ provides documented proof that the manufacturing environment is controlled, repeatable, and auditable.
IQ is essential because it:
- Prevents undocumented equipment substitution
- Establishes a validated baseline for audits
- Supports long-term traceability for regulated devices
- Reduces regulatory risk during design transfer and scale-up
Without IQ, even functional medical PCBAs may be considered non-compliant during audits.
What Are the Risks of Skipping or Weak IQ in Medical PCBA?
Incomplete or missing IQ documentation can lead to:
- Audit nonconformities
- Delayed supplier qualification
- Re-validation requirements
- Increased regulatory exposure for OEMs
Even when PCBAs pass electrical testing, lack of IQ can result in formal non-acceptance during audits or regulatory reviews.
How Does Best Technology Implement IQ for Medical PCBA Projects?
Best Technology applies a structured IQ approach that includes:
- Approved equipment lists and installation records
- Verification of utilities and environmental conditions
- Calibration status confirmation
- MES and traceability validation
- Controlled documentation aligned with medical audits
This ensures a repeatable and defensible foundation for all downstream validation activities.
What is the Relation Between IQ and OQ?
IQ confirms that the manufacturing environment is correctly installed, but it does not validate process performance.
Once installation is confirmed, manufacturers must demonstrate that assembly processes operate reliably within defined limits. This is achieved through Operational Qualification (OQ).
Related reading:


