Why choose copper base plate for medical device? Let’s discover benefits, application, design guide and manufacturing process for medical copper base plate.
Are you troubled with these issues?
- Is your device’s performance degraded due to poor heat dissipation from traditional substrates?
- During high-frequency signal transmission, does impedance mismatch on copper substrates cause signal distortion?
- In miniaturized designs, can existing substrates meet the requirements for ultra-thin 0.3mm wiring?
EBest Circuit (Best Technology) can provide solutions and service and solutions:
- High Thermal Conductivity Copper Substrate: Thermal conductivity reaches 400W/mK (60% higher than conventional substrates), passing JEDEC JESD51-14 thermal testing.
- Precise Impedance Control: Surface roughness Ra ≤ 0.8μm (reducing signal loss by 30%), supporting stable transmission at 10GHz+.
- Ultra-Thin Laser Processing: Line width/space 75μm (compared to the industry standard of 100μm), achieving mass production of ultra-thin 0.2mm substrates.
Welcome to contact us if you have any inquiry for copper base plate: sales@bestpcbs.com.
Why Choose Copper Base Plate for Medical Device?
Reasons why choose copper base plate for medical device:
Excellent thermal conductivity ensures stable equipment operation
- Copper substrates have a thermal conductivity of up to 401 W/(m·K), far exceeding the 0.3-0.4 W/(m·K) of traditional FR-4 substrates. This makes them ideal for high-power medical devices such as surgical lights, laser therapy devices, and power modules. Efficient heat dissipation prevents component overheating, ensuring stable operation and minimizing the risk of failure.
Natural antibacterial and antiviral properties reduce the risk of hospital-acquired infections
- Copper surfaces have a broad-spectrum bactericidal effect, killing 99.9% of pathogens such as E. coli within 2 hours and significantly inhibiting viruses such as influenza. In hospitals, copper substrates used on high-touch surfaces such as door handles and faucets can reduce hospital-acquired infections (HCAIs) by 40%-70%, meeting the high sterile environment requirements of medical institutions.
Excellent Corrosion Resistance and Biocompatibility
- The copper substrate is ISO 10993 and USP Class VI certified, demonstrating low cytotoxicity, non-sensitization, and resistance to long-term immersion in body fluids. This corrosion resistance stems from the dense cuprous oxide protective layer formed on the copper surface. Even in complex environments like medical gas pipelines, it resists corrosion from body fluids, disinfectants, and gases, ensuring long-term safe operation of the device.
Precision Machining and Electromagnetic Shielding
- The copper substrate’s etching process enables high-density wiring, supporting complex circuit designs and making it suitable for high-frequency medical electronic equipment such as MRI and ultrasound equipment. Furthermore, copper’s conductivity makes it an ideal material for electromagnetic shielding, effectively blocking external electromagnetic interference and ensuring accurate signal transmission.

Applications of Copper Base Plate in Medical Device
- Medical Imaging Equipment Cooling – X-ray tube cooling systems for CT and MRI equipment.
- Laser Therapy Equipment – Dermatology lasers, surgical laser heads.
- Implantable Electronics – Pacemakers, Neurostimulators.
- Endoscopic Microcircuits – Gastrointestinal endoscopes, laparoscopic sensors
- High-Frequency Surgical Instruments – Electrosurgical units, radiofrequency ablation electrodes
- Wearable Medical Devices – Holter patches, blood glucose monitors
Medical Device Copper Base Plate Design Guide
1. Requirements Analysis and Specification Definition
- Clear Application Scenario: Differentiate between implantable devices (e.g., pacemakers) and external devices (e.g., CT scanners), and determine core requirements such as high-frequency signal transmission, heat dissipation, and biocompatibility.
Parameter Definitions:
- Thermal conductivity ≥ 400 W/m·K (high-power devices).
- Temperature resistance range -40°C to 150°C (compatible with sterilization processes).
- Compliance with ISO 10993-5 biocompatibility standards (implantable devices).
2. Material Selection and Pretreatment
Copper Substrate Type:
- High-frequency devices: Rolled copper (surface roughness ≤ 1.5 μm) to reduce signal loss.
- High-heat dissipation requirements: Thermoelectrically isolated copper substrate with a thermal resistance ≤ 0.8°C/W.
- Surface Treatment: Gold plating (thickness ≥ 0.25 μm) or titanium nitride coating, passing a salt spray test for ≥ 500 hours.
3. Stack-up Design and Routing Planning
Layer Allocation:
- Basic Equipment: 4 layers (signal-ground-power-signal).
- High-End Imaging Equipment: 6 or more layers, with alternating high-frequency signal and ground planes.
Line Width Control:
- Conventional circuits: Line width ≥ 0.1mm, miniaturized to 0.05mm for implantable devices (impedance matching ±5%).
4. Thermal Management Solution Design
- Thermal Path Optimization: A stepped copper substrate design reduces heat density in CT equipment by 40%.
- Heat Dissipation Verification: ANSYS simulation demonstrates full-load temperature rise ≤ 15°C (IEC 60601-1 standard).
5. EMC and Signal Integrity Assurance
- Shielding Design: Ground planes are added on both sides of sensitive signal layers, ensuring EMI interference of MRI equipment ≤ 10μT.
- High-Frequency Performance: Dielectric loss Df < 0.003 (10GHz band).
6. Mechanical Structure Verification
- Strength Testing: Surgical instrument substrates must have a tensile strength ≥ 300MPa (ASTM B209).
- Flexible Design: Wearable devices use 1.2mm thick rolled copper with a bend radius of ≥5mm.
7. Sterilization and Environmental Compatibility Testing
- Sterilization Resistance: Passed 500 cycles of 134°C steam sterilization, maintaining an insulation resistance of 10¹⁰Ω.
- Humidity Protection: Select boards with a water absorption of ≤0.02% to prevent leakage.
8. Prototyping and Functional Verification
- Rapid Prototyping: Laser processing for small-batch prototypes, and etching for mass production (reducing costs by 40%).
- Test Point Design: Reserve key signal test points to ensure 100% functional coverage.
9. Compliance Certification and Documentation Preparation
- Standard Compliance: FDA and ISO 13485 certified, with a complete material report provided.
- Usability Documentation: Includes user scenario simulations and environmental risk assessments.
10. Mass Production and Continuous Optimization
- Process Standardization: Etching is used for mass production, with a tolerance control of ±0.02mm.
- Feedback and Iteration: Collect clinical data to optimize designs (e.g., the corrosion rate of a pacemaker substrate was reduced to 0.08μm/year).
11. Design Considerations
- Biocompatibility Priority: Implantable devices must pass ISO 10993-5 cytotoxicity testing to prevent excessive copper ion release.
- High-Frequency Signal Isolation: MRI equipment wiring spacing must be ≥ 2x the line width to prevent crosstalk.
- Sterilization Compatibility: Avoid the use of halogen-containing materials to prevent post-sterilization corrosion.
- Cost Control: Laser processing is preferred for small batches, while etching is used for mass production (reducing costs by 40%).
- Failure Prevention: Replacing right-angle traces with 45° chamfers increases fatigue life by 3 times (measured data).

Medical Device Copper Base Plate Manufacturing Processes
1. Material Preparation and Pretreatment
- 99.9% high-purity electrolytic copper plates are selected and ultrasonically cleaned to remove surface oxide and impurities.
- Thickness calibration is performed using a CNC milling machine (tolerance controlled within ±0.02mm).
2. Graphic Design and Transfer
- Circuit patterns are designed using CAD software and photolithography masks are generated.
- After applying photoresist, the circuit pattern is transferred using a UV exposure machine.
3. Etching and Forming
- Selective etching is performed using an ammonium chloride + hydrogen peroxide etchant.
- Laser cutting and forming (accuracy of ±0.1mm) is achieved with burr-free edges.
4. Surface Treatment
- Electroless Nickel Ion Gold (ENIG) technology ensures solderability and corrosion resistance.
- Plasma cleaning removes micron-sized residues and improves surface energy.
5. Precision Machining
- Micro-hole machining is performed using a CNC drilling machine (adjustable hole diameter 0.3-3.0mm).
- Flatness is checked using a coordinate measuring machine (required to be ≤0.05mm/m).
6. Quality Verification
- Salt spray testing verifies corrosion resistance (meets ASTM standards). B117 standard).
- Electrical performance test (insulation resistance ≥ 100MΩ, withstand voltage test 1500V/1min).
7. Sterilization and packaging
- Ethylene oxide sterilization (to ensure biocompatibility).
- Anti-static vacuum packaging + humidity indicator card for dual protection.
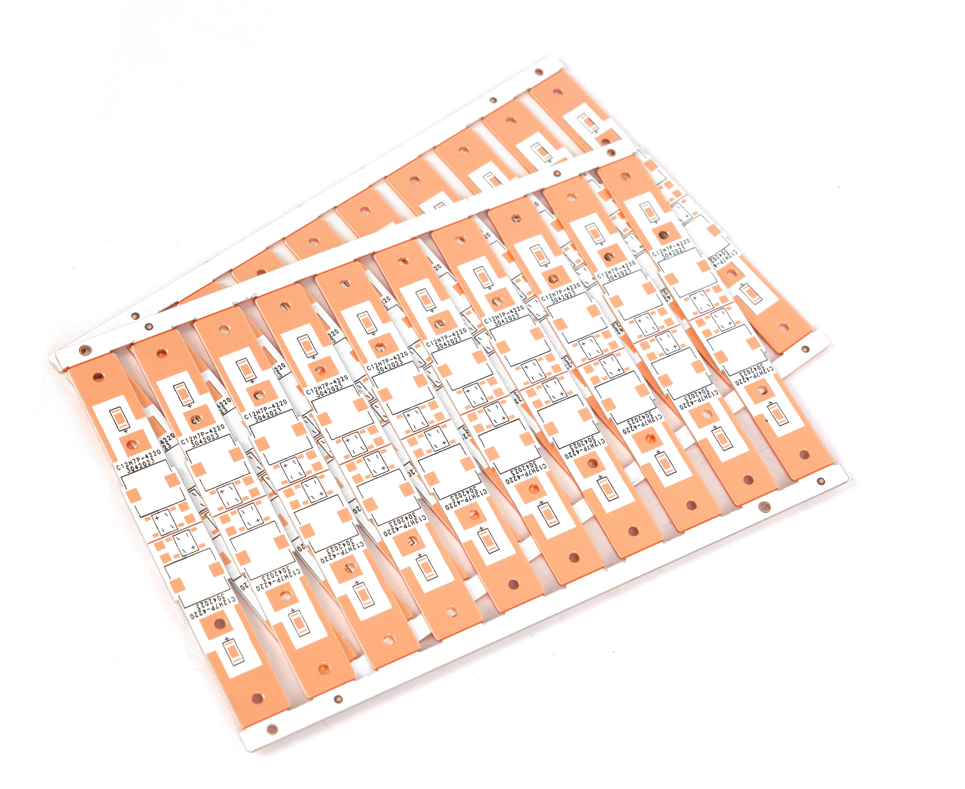
Future Trend of Copper Base Plate in Medical Device
Breakthroughs in High-Precision Microfabrication Technology
- Laser direct writing will replace traditional etching processes, achieving line accuracy of <5μm (meeting the requirements of minimally invasive devices such as neural electrodes).
- The rise of 3D-printed copper substrate technology supports the integrated molding of complex intraluminal structures (such as thermal management modules for vascular interventional catheters).
Upgraded Biocompatible Surface Treatment
- The widespread adoption of graphene-copper composite coating technology increases antibacterial rates to 99.9% while maintaining excellent conductivity.
- Bionic micro-nanostructure surface treatment reduces platelet adhesion (applicable to key components of extracorporeal circulation equipment).
Intelligent Function Integration
- Embedded fiber optic sensors monitor substrate temperature and strain in real time (data is wirelessly transmitted to the medical IoT platform via LoRa).
- AI-driven thermal simulation and optimized design improves heat dissipation efficiency by over 40% (reducing the failure rate of high-power medical laser equipment).
Sustainable Manufacturing Transformation
- Electrolytic copper foil thickness reduced to 18μm (material utilization increased by 30%, meeting new EU carbon footprint requirements).
- Cyanide-free electroplating completely replaces traditional gold plating, reducing wastewater treatment costs by 60%.
Expanded Application Scenarios
- Wearable Medical Devices: Flexible copper substrate with a bending radius of <3mm (suitable for smart patch-type ECG monitoring devices).
- Surgical Robotics: Nanoporous copper substrates facilitate heat dissipation in joint modules (continuous operating temperature fluctuations controlled to ±0.5°C).
Our Medical Copper Base Plate Case Studies
At EBest Circuit (Best Technology), we specialize in medical device copper base plate manufacturing, with over 19 years of industry experience. We have successfully completed over 300 medical device projects (including core components for MRI and CT imaging systems and surgical robots), and our entire product line is ISO 13485 certified. We offer an end-to-end solution, from circuit design optimization to 24-hour rapid prototyping to fully automated mass production and modular assembly. We look forward to providing highly reliable copper PCB customization services for your project. Here is a photo of copper base plate we made before:
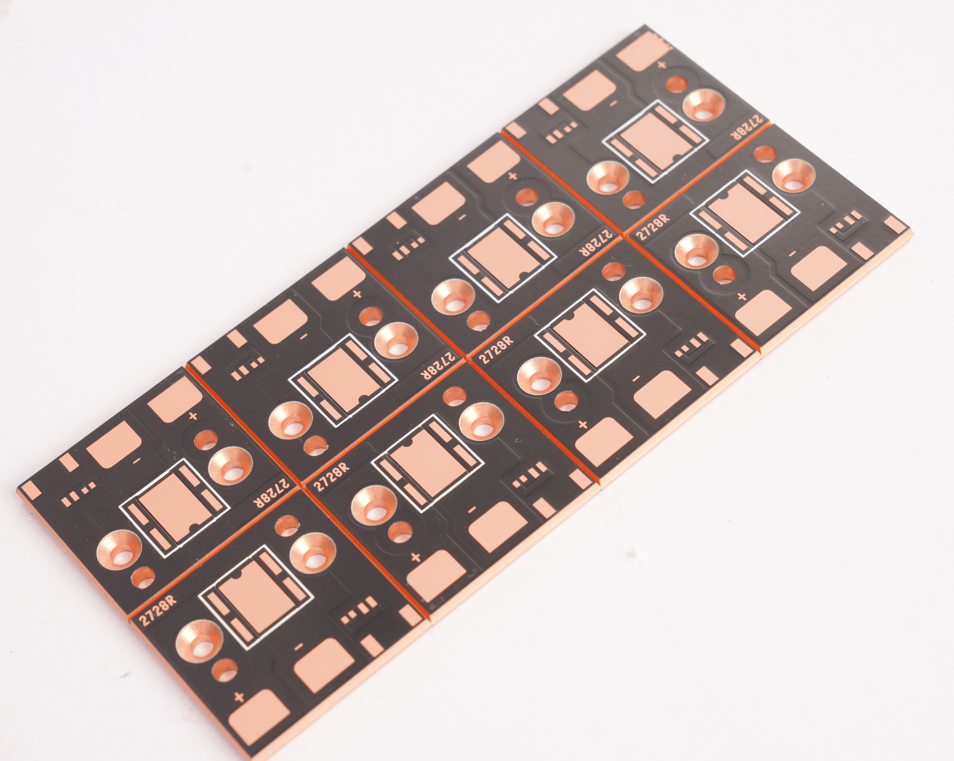
Attached is technical specification for medical device copper base plate
| Parameter Category | Technical Specifications |
| Product Name | High-Thermal-Conductivity Medical Copper Base Plate |
| Base Material | 99.9% Pure Oxygen-Free Copper (OFC), compliant with ASTM B152 |
| Thickness Range | 0.3mm–3.0mm (±0.03mm tolerance), customizable stepped thickness available |
| Solder Mask | Liquid Photoimageable (LPI) solder mask, withstands 260°C/10s, UL certified |
| Legend Printing | White anti-etching ink, line width ≥0.15mm, FDA 21 CFR Part 175.300 compliant |
| Surface Finish | Electroless Nickel Immersion Gold (ENIG), Ni layer 3–6μm, Au layer 0.05–0.1μm |
| Process | Laser micro-drilling (±0.05mm tolerance) + plasma cleaning |
| Application | High-frequency surgical electrodes (conductivity ≥58 MS/m) |
Why Choose EBest Circuit (Best Technology) as Copper Base Plate Manufacturer?
Reasons why choose us as copper base plate manufacturer:
- Global Medical Certification Guarantee: ISO13485 & ISO9001 certified, covering the entire design, production, and delivery process, ensuring compliance with international standards such as FDA/CE.
- Customized Thermal Management Experts: 19 years of experience optimizes boss design and metal layer thickness, resulting in thermal resistance as low as 0.5°C/W, suitable for high-power devices (such as laser surgical systems).
- Transparent Cost: Copper substrate solutions reduce costs by 30%, ENEPIG/hard gold surface treatments balance corrosion resistance and cost-effectiveness, and platinum-iridium alloy coatings are available for implantable devices.
- 24-Hour Rapid Prototyping: Combined with free Design for Everything (DFM) analysis, design flaws (such as insufficient creepage distance) can be identified early, shortening development cycles by 50% to meet urgent needs.
- 100% Automated Quality Inspection: AOI inspection covers all bulk orders, ensuring zero-defect delivery, reducing manual errors, and ensuring high reliability of medical devices.
- Stable Supply Chain Support: Long-term inventory of key materials (medical copper foil and ceramic substrates) mitigates shortages and ensures delivery consistency. One-stop, full-cycle service: Integrating design, processing, and thermal management technologies, simplifying the supply chain, reducing project risk by 40%, and accelerating time to market.
- High-reliability design standards: Dual redundancy for critical circuits, achieving an MTBF of 80,000 hours, combined with ANSYS simulation verification, meets diagnostic-level accuracy requirements.
How to Get a Quote for Copper Base Project?
Materials Required for Quotation
Project Basic Information
- Application Scenario (e.g., CT Scanner, Laser Therapy Device)
- Copper Substrate Dimensions (Length × Width × Thickness, in mm)
- Expected Purchase Quantity (Batch/Prototype)
Technical Specifications
- Copper Foil Thickness (e.g., 1oz/35μm or 3oz/105μm)
- Number of Layers (Single/Dual/Multi-Layer)
- Surface Treatment (Electroless Nickel Gold Plating/OSP/Tin Plating)
- Special Requirements (e.g., Thermoelectric Separation Structure, Biocompatible Coating)
Certification and Compliance Requirements
- Target Market Certifications (FDA/CE/ISO13485)
- Industry-Specific Standards (e.g., ISO10993 for Implantable Devices)
Why Choose EBest Circuit (Best Technology)?
- 19 years of experience in medical devices: Expertise in high-power, high-frequency, and implantable device requirements.
- 24-hour fast quote: Accurate quote within one business day of submitting your request.
- Free DFM optimization: Avoid 90% of potential issues during the design phase, reducing board revision costs.
Welcome to contact us if you have any request for copper base plate: sales@bestpcbs.com.
Tags: copper base, copper base plate


